Biological Oxidations Often Involve Which of the Following
In several steps Acetyl-CoA is oxidized releasing CO 2. Reduction potentials measure affinity for electrons.
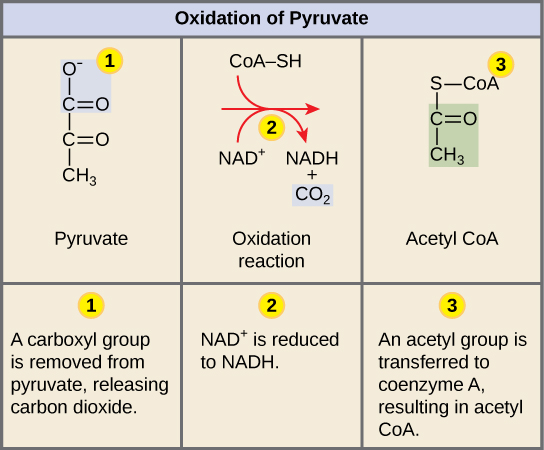
Pyruvate Oxidation Cellular Respiration Article Khan Academy
Many vitamins are important for metabolism because they act as _____.

. Enzymes are complex biological catalysts and are critical to life. Which of the following compounds is NOT an enzyme. All of the following are associated with the process of glycolysis.
Cellular oxidation of glucose to carbon dioxide requires specialized electron carriers. A few types of coenzymes and proteins serve as universal electron carriers. Different enzymes associated with biological oxidation are.
NAD FAD and NADP are all examples of _____. NAD usually binds reversibly to enzymes and can thus be considered a substrate. How ever there have also been.
Photosynthetic Because they involve the removal of both electrons and protons biological oxidations are often referred to as a. 2 NADH are also made. If a species gains electrons it is undergoing a reduction.
An oxidation is defined as loss of electrons in the course of a chemical reaction. 2 ATP are made. To a detailed report on the pio neer work of his group on liver alcohol dehydrogenase.
2 Conversion of NAD1 to NADH and H1 is an example of a. Biological oxidations often involve which of the following. BIOLOGICAL OXIDATIONS catalyzed exchange of C-l and C-2 may occur between fructose and pentu lose phosphates so as to increase the label in the former molecule.
Biological oxidations often involve dehydrogenation. There has been however considerable activity in pushing for ward those salients that have already been established in the past with the result that some important new ground has been gained. Standard reduction potentials can be used to calculate free energy change.
It involved the Krebs cycle. Sulfanilamide is a drug that interferes with bacterial enzymes in which of the following ways. B-galactosidase Cellulase Dehydrogenase Coenzyme A Sucrase Coenzyme A 19.
2 GTP ATP are made. Biological oxidations often involve which of the following. The loss of a hydrogen atom the gain of an oxygen atom the gain of an electron the loss of an oxygen atom A ribozyme is an enzyme made out of.
Oxidoreductase enzymes and biological redox cosubstrates. BALL Department of Biological Chemistry Harvard Medical School Boston. In contrast FAD is tightly bound to enzymes and is thus considered a cofactor.
Biological oxidations are often called dehydrogenation reactions because they involve the loss of a hydrogen atom. Oxidation of carbohydrates or sugars is the primary source of bioenergy multiple enzymes are involved for the many steps A carbohydrate basically has a formula with one OH per carbon C6H12O6 O2 enzymes 6 CO2 6 H2O energy s ugars. BRITTON CHANCE AND LUCILE SMITH Johnson Research Foundation University of Pennsylvania Philadelphia Pennsylvania This year a particular emphasis on the mechanism of enzymatic action in biological oxidations is quite appropriate because of the unusually large number of books and reviews that have appeared on the general topic of oxidative enzymes 1 to 5.
Most biological oxidations are a. Photosynthetic Because it is a degradative pathway that also generates biosynthetic precursors the citric acid cycle is an 4. Biological oxidations are often _____ Oxidation-Reduction Reactions In biological systems electrons and protons are removed at the same time.
According to the scheme shown in Reaction 1 a combination of dilution by endogenous dihydroxyacetone phosphate and an exchange catalyzed by transketolase could produce a hexose unit with. Biological oxidation is catalysed by enzymes which function in combination with coenzymes andor electron carrier proteins. 2 NADH are made.
Enzymatic reactions reach a saturation point when the active sites of all enzymes present are occupied by substrate molecules. Involves the breaking of glucose to 2 pyruvate. 6 NADH and 2 FADH 2 are made.
Oxidation-reduction or redox reactions are a very large class of chemical reactions in which both oxidation and reduction necessarily occur. These are nicotinamide adenine dinucleotide NAD and NADH and flavin adenine dinucleotide FAD and FADH 2. Most oxidations of chemicals are catalyzed by cytochrome P450 P450 CYP enzymes which generally utilize mixed-function oxidase stoichiometry utilizing pyridine nucleotides as electron donors.
NAD PH O 2 R NAD P RO H 2 O where R is a carbon substrate. These enzymes catalyse the removal of hydrogen from the substrate and add it to another substance thus bringing about oxidation reduction reaction. Massachusetts No new salients have been described in this field within the last year.
Biological Oxidations and Reductions Biological Oxidations and Reductions Stern K G 1940-07-01 000000 Laboratory of Physiological Chemistry Yale University School of Medicine New Haven Connecticut Work in the field of biological oxidation during the year 1939 has made notable advances at many points along a far-flung front. Two compounds are widely used in biological oxidations and reductions. Sulfanilamide is a drug that interferes with bacterial enzymes in which of the following ways.
The loss of a hydrogen atom. Some Biological Alcohol Oxidations Not for Test 1. Biological oxidations often involve which of the following.
Pyruvate is transported into the mitochondria and converted to Acetyl-CoA CO 2 is released. Which of the following types of transport involves the expenditure of energy and special membrane proteins. Equivalent to a hydrogen atom.
Often on standardized tests. The Boyer-Theorell effect a shift of the maximum to shorter wavelengths combined with an in crease in intensity of the fluorescence of bound DPNH as compared to free DPNH has been shown to occur with yeast alcohol dehydrogenase 49 50 beef heart lactic dehydrogenases.

Pfk 2 Fbpase 2 Control Of Glycolysis And Gluconeogenic Pathways Download Scientific Diagram Biochemistry Notes Biology Classroom Glycolysis Pathways

Ozonolysis Kmno4 Oxidation Terminal Alkenes Chemistry Chemistry Lecture Science Education

Chemical Reactions Part I Chemistry Lessons Chemistry Classroom Chemical Reactions
Belum ada Komentar untuk "Biological Oxidations Often Involve Which of the Following"
Posting Komentar